Deionization
Large cation/anion ion exchangers used in demineralization of boiler feed water.
Deionized water (DI water, DIW or de-ionized water), often confused with demineralized water / DM water, is water that has had almost all of its mineral ions removed, such as cations like sodium, calcium, iron, and copper, and anions such as chloride and sulphate. Deionization is a chemical process that uses specially-manufactured ion exchange resins, which exchange hydrogen and hydroxide ions for dissolved minerals, and then recombine to form water. Because most non-particulate water impurities are dissolved salts, deionization produces a high purity water that is generally similar to distilled water, and this process is quick and without scale buildup. However, deionization does not significantly remove uncharged organic molecules, viruses or bacteria, except by incidental trapping in the resin. Specially made strong base anion resins can remove gram-negative bacteria. Deionization can be done continuously and inexpensively using electro deionization.
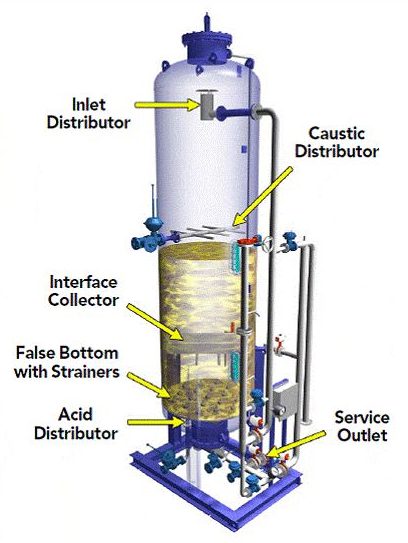
Three types of deionization exist: co-current, counter-current, and mixed bed.
Co-current deionization
Co-current deionization refers to the original downflow process where both input water and regeneration chemicals enter at the top of an ion exchange column and exit at the bottom. Co-current operating costs are comparatively higher than counter-current deionization because of the additional usage of regenerates. Because regenerate chemicals are dilute when they encounter the bottom or finishing resins in an ion exchange column, the product quality is lower than a similarly sized counter-flow column.
The process is still used and can be maximized with the fine tuning of the flow of regenerates within the ion exchange column.